2-bromoacetophenone
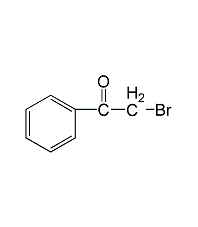
Structural formula
| Business number | 01FZ |
|---|---|
| Molecular formula | C8H7BrO |
| Molecular weight | 199.05 |
| label |
Bromoacetophenone, 2-Bromo-1-phenylethanone, ω-bromoacetophenone, Benzoyl Methyl Bromide, Bromoacetophenone, Alpha-bromoacetophenone, 2-Bromoacetophenone, Phenacyl bromide, Bromoacetylbenzene, ω-Bromoacetophenone, Aromatic aldehydes, ketones and their derivatives |
Numbering system
CAS number:70-11-1
MDL number:MFCD00000195
EINECS number:200-724-9
RTECS number:None
BRN number:606474
PubChem number:24887194
Physical property data
1. Properties: White needle-like crystals precipitated from ethanol
2. Density (g/mL, 25/4℃): 1.476
3. Relative vapor density (g/mL, air=1): Uncertain
4. Melting point (ºC): 48-51
5. Boiling point (ºC, normal pressure): Uncertain
p>
6. Boiling point (ºC, 18mmHg): 135
7. Refractive index: 1.709 (15℃)
8. Flash point (ºF): >230
9. Specific rotation (º): Uncertain
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure ( kPa, 25ºC): Uncertain
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Oil-water (octanol/water) partition coefficient relationship Value: Uncertain
17. Explosion upper limit (%, V/V): Uncertain
18. Explosion lower limit (%, V/V): Uncertain
19. Solubility: Easily soluble in ether, benzene and chloroform, soluble in ethanol and hot petroleum ether, insoluble in water
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 44.01
2. Molar volume (cm3/mol): 134.1
3. Isotonic specific volume (90.2K ): 344.5
4. Surface tension (dyne/cm): 43.5
5. Polarizability (10-24cm3): 17.44
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 2.5
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 2
6. Topological molecular polar surface area (TPSA): 17.1
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 116
10. Number of isotope atoms : 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the chemical bond configuration Number of centers: 0
14, Number of uncertain chemical bond stereocenters: 0
15, Number of covalent bond units: 1
Properties and stability
This product is toxic and has strong tear-inducing properties. It can burn when exposed to open fire and emit a large amount of tear-inducing vapor when exposed to high heat.
Storage method
This product should be sealed and stored away from light.
Packaged in glass bottles with wooden boxes lined with padding or iron drums. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Store and transport separately from oxidants and edible raw materials. When transporting, load and unload gently to prevent damage to the container.
Synthesis method
Originated from bromination of acetophenone. Add water and benzene into the reaction pot, stir, quickly add bromine at 25°C, slowly raise the temperature to 55-60°C, react for 10-20 minutes, add ice water, cool to 0-5°C, filter, and use for filter cake Wash with ice water and spin dry to obtain bromoacetophenone. The yield is 92%. During the above reaction, a thunderous sound can be emitted from the reaction pot. It is also possible to react acetophenone with bromine dropwise in an ether solvent, and add anhydrous aluminum trichloride to the reaction solution. This method obtains crude product with a yield of 88-96%. In addition, acetophenone and bromine can also be produced by the interaction of acetophenone and bromine in the presence of aluminum oxide, with a yield of 94%.
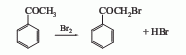
Purpose
This product is a raw material for organic synthesis and an intermediate for medicines and dyes. In the pharmaceutical industry, it is used to inhibit blood speed, etc., and can be synthesized with thiourea to synthesize α-amino-4-phenylthiazole. Determination of hydroxy acids. Organic Synthesis.
extended-reading:https://www.newtopchem.com/archives/40004extended-reading:https://www.newtopchem.com/archives/category/products/page/179extended-reading:https://www.newtopchem.com/archives/44882extended-reading:https://www.bdmaee.net/dabco-dc1-delayed-catalyst-dabco-dc1-delayed-strong-gel-catalyst-dabco-dc1/extended-reading:https://www.newtopchem.com/archives/category/products/page/49extended-reading:https://www.newtopchem.com/archives/category/products/page/24extended-reading:https://www.cyclohexylamine.net/high-quality-n-dimethylaminopropyldiisopropanolamine-cas-63469-23-8-n-3-dimethyl-amino-propyl-n-n-diisopropanolamine/extended-reading:https://www.newtopchem.com/archives/1888extended-reading:https://www.morpholine.org/cas-63469-23-8/extended-reading:https://www.newtopchem.com/archives/44759


